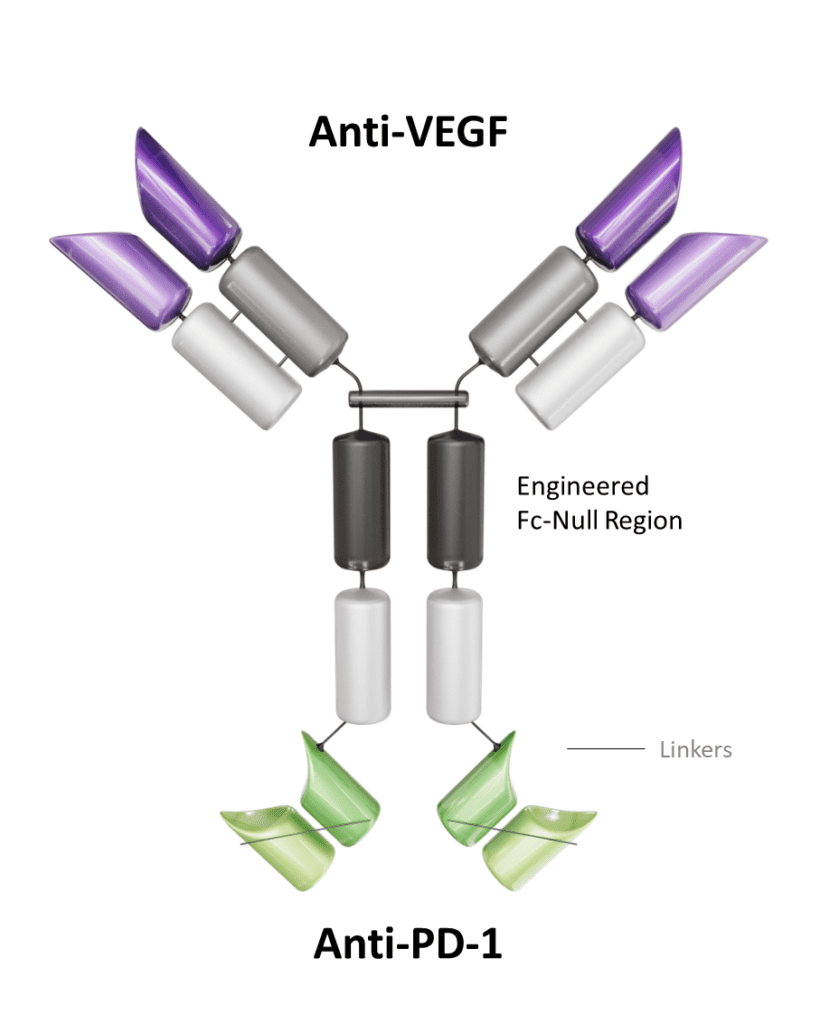
Ivonescimab Overview
Designed to potentially improve the balance of anti-tumor activity and safety1,2
Ivonescimab is the most advanced PD-1/VEGF bispecific antibody in clinical development in the U.S. & EU* and is an investigational therapy that is not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA). It brings two validated mechanisms in oncology3-5 into ONE novel tetravalent molecule. Ivonescimab simultaneously engages both PD-1 & VEGF. To-date over 1,600 patients have been treated with ivonescimab in clinical trials in China and Australia. Summit is actively recruiting 100+ patients in the U.S., Canada and Europe; the overall study will include over 400 patients worldwide.
Mechanism of Action (MOA) based on in vitro studies
Simultaneous interaction of PD-1 & VEGF blockades have the potential to drive synergistic anti-tumor activity1,2,6
Inhibiting VEGF can help improve the effect of immunotherapy by modulating the tumor microenvironment (TME).6
Enhancing the PD-1 blockade helps activate T cells.6
COOPERATIVE BINDING
Increased Binding Strength (Affinity)
Presence of VEGF increases PD-1 binding strength by >18X6
Presence of PD-1 increases VEGF binding strength by >4X6
Increased Binding of T Cells
VEGF dimer leads to potential interconnection or daisy chaining of multiple ivonescimab molecules, which may lead to increased binding of T cells6
Tumor Microenvironment
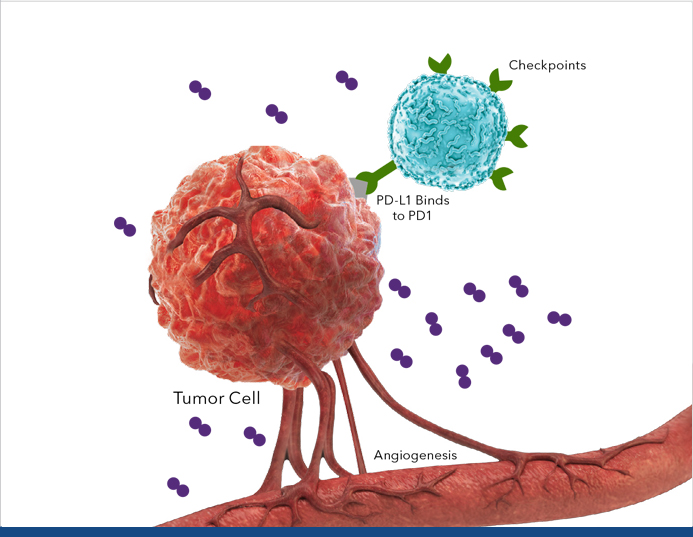
Images for illustrative purposes only.
Tumor Microenvironment with Ivonescimab Cooperative Binding
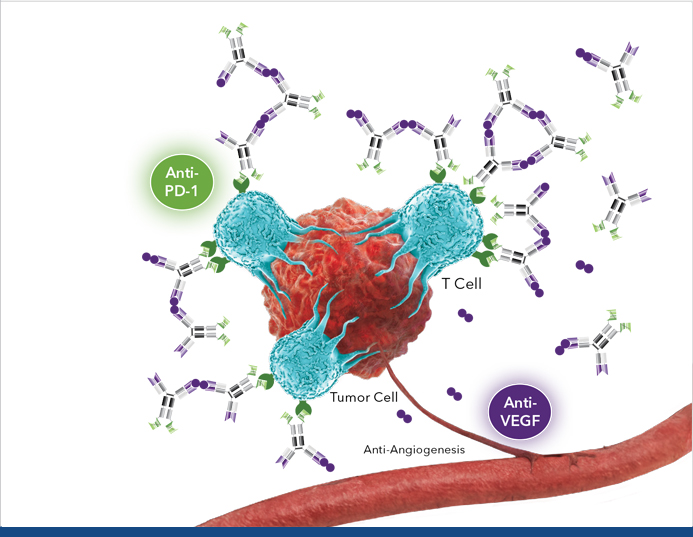

VEGF Dimer

PD-1 Receptor in T Cell
Potential Safety Benefits
Ivonescimab has the potential to accumulate in the Tumor Microenvironment where there are higher levels of PD-1 and VEGF vs. healthy tissue.1,2,6
Half-life (T1/2) of 6-7 days6
Provides blockade of both PD-1 and VEGF targets with its affiliated clearance, which could potentially lead to a favorable safety profile1,2
Clinical Trials
For additional information on the HARMONi or the HARMONi-3 clinical trials, please visit clinicaltrials.gov or contact medinfo@smmttx.com
References
- Zhao Y. et al. eClinicalMedicine. 2023; 3(62): 102106.
- Wang L, et al. J Thorac Oncol. 2024 Mar;19(3):465-475.
- Manegold C, et al. J Thorac Oncol 2017;12(2):194-207.
- Pardoll, D. Nat Rev Cancer 2012;12(4):252-64.
- Tamura R, et al. Med Oncol 2020;37(1):2.
- Zhong T, et al. AACR-NCI-EORTC International Conference 2023.
Poster #B123, Abstract #35333, Boston, MA, USA.
Ivonescimab is an investigational therapy that is not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA).
*There are no known PD-1-based bispecific antibodies approved by the U.S. Food and Drug Administration (“FDA”) or the European Medicines Agency (“EMA”).