
HARMONi Phase 3 Clinical Trial
Patients with EGFR+ NSCLC Who Have Progressed After 3rd Generation EGFR-TKI (osimertinib)
NCT06396065: Click to view on ClinicalTrials.gov
A randomized, double-blind, multi-center, phase 3 study of SMT112 or placebo combined with pemetrexed and carboplatin in patients with EGFR-mutant locally advanced or metastatic non-squamous NSCLC who have progressed on EGFR-TKI treatment.
HARMONi Study Schema
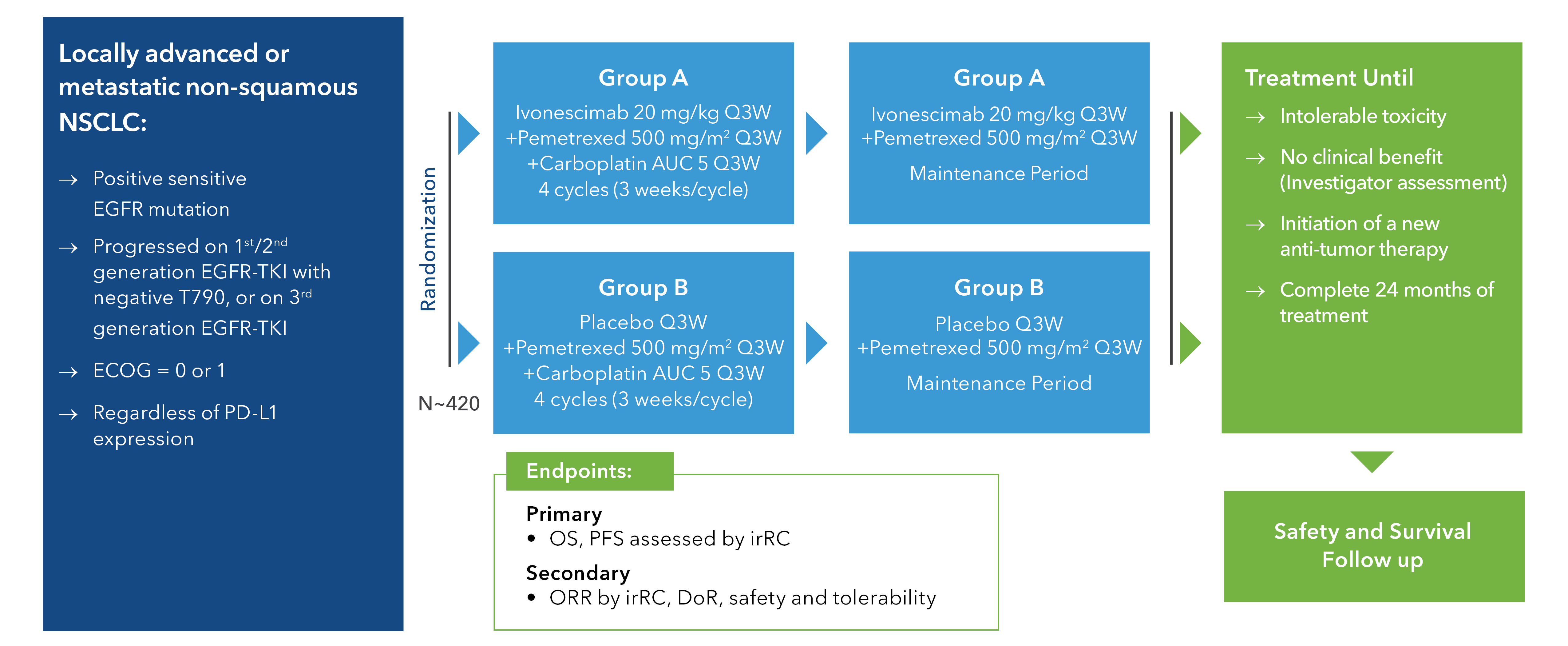
Key Eligibility Criteria
- Expected survival ≥3 months
- Locally advanced (Stage IIIB/IIIC) or metastatic (Stage IV) NSCLC
that has progressed on 3rd generation EGFR-TKI (e.g., osimertinib) - At least 1 measurable noncerebral per RECIST v1.1 lesion
- Adequate organ and hematologic function
- Prior treatment with one non-EGFR therapy is allowed (i.e amivantamab, REQORSA, etc,).
Prior treatment with immune checkpoint inhibitors, anti-angiogenic therapy and
chemotherapy (including ADCs) remain exclusionary - Tumor does not surround important blood vessels, have obvious necrosis and/or cavitation
or invade the surrounding vital organs and blood vessels - No symptomatic metastases of the central nervous system
- No history of esophageal gastric varices, severe ulcers or wounds that do not heal
- No history of severe bleeding tendencies or coagulopathy, or hemoptysis within last 4 weeks
Ivonescimab is an investigational therapy that is not approved by any regulatory authority other than China’s National Medical Products Administration (NMPA).
For additional information on the HARMONi Clinical Trial, please contact medinfo@smmttx.com
